Aphasia Access FAQ on Research
#1: Getting Started
Aphasia Access' Education and Research Committee, is committed to expanding the base of quality research available on the Life Participation Approach to Aphasia. To that end, the committee has begun releasing a series of "Frequently Asked Questions" designed to assist those new to conducting research studies that may be appropriate for peer review at some point.
Below is the first in the FAQ series - Getting Started. Questions may be directed to [email protected].
Please Note: Aphasia Access does not offer this material in lieu of expert advice.You are encouraged to familiarize yourself with the legal, ethical, and scientific practices that apply in your local setting or practice. If you have a research project in mind but have limited experience with research ethics or design, we recommend you connect with someone who has the relevant expertise.
Q. I hear a lot of talk about the complexities of getting “IRB approval” for research. What exactly is an IRB?
All human subjects research is subject to local, state, and federal regulations governing the safe and ethical treatment of participants. All universities, hospitals, and other entities that conduct grant-supported research have boards or committees that review proposed research projects to insure compliance with the relevant laws and statutes. Institutional Review Boards (IRBs) or Research Ethics Boards (REPs) are the most common such committees.
Q. As a free-standing aphasia center, our activities do not come under the auspices of an IRB or other research ethics committee. What are my options?
If your research involves collaboration with a hospital- or university-affiliated researcher, your collaborator’s research ethics committee should be able to provide the necessary oversight. If you are not currently engaged in such a collaboration, it would be advisable to seek a local institutional or commercial board that could provide the necessary oversight. Another option is to form one’s own research eithics committee before planning a research project.
Q As a clinician in an aphasia center not currently affiliated with an IRB, when and how do I get started learning about research ethics and review?
If you are thinking of gathering outcomes or other data from the participants in your program that might one day be published, or if you plan to apply for grant funds to gather such data, the time is right to familiarize yourself with some of the many research-ethics training resources available online. For example, the U.S. National Institutes of Health offers a free training course at https://phrp.nihtraining.com/index.php. Also, many universities have training videos posted on YouTube.
Q. My aphasia center routinely gathers data about participant satisfaction. After each program module, we obtain information about what the participants liked about the module, what they didn’t like, and what changes they would like to see. Does this qualify as research?
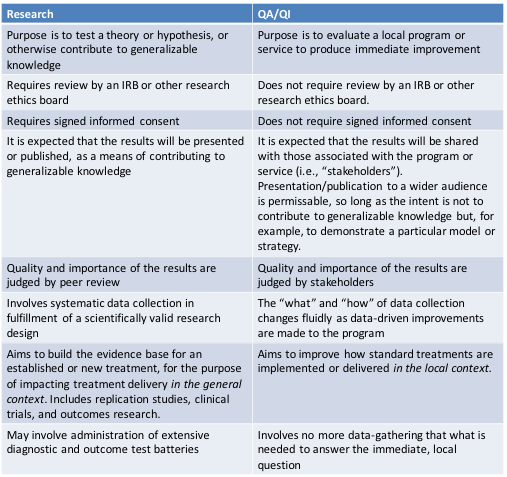
It depends. Research is systematic data collection aimed at contributing to generalizable knowledge by answering a question or testing a hypothesis of broad interest to the field. Research is different from data gathering for purposes of Quality Assessment and Quality Improvement (QA/QI). QA/QI, also known as program evaluation, is aimed at producing immediate improvement in a local program or model. If your center’s purpose in measuring user satisfaction is solely to improve its program offerings, the activity counts as QA/QI, not research. But if the activity is aimed at a broader scientific purpose, such as building the evidence base for an aphasia treatment or outcome measure, then it probably does qualify as research.
Q. Do QA/QI activities require approval from an IRB or other research ethics board?
No. An aphasia center’s QA/QI activities are generally overseen by its Board of Directors or institutional administrators. They, along with the center’s consumers and clinicians, are stakeholders in the program’s success and the ones responsible for QA/QI oversight. IRB oversight is usually reserved for human subjects research. The IRB is not concerned with program improvements but rather with the ethical treatment of the human subject participants, including their voluntary participation and rights of confidentiality.
Q. Does data-gathering for QA/QI require written informed consent from participants.
No. However, in the aphasia center context, it is considered good practice to involve participants in QA/QI decision-making and to inform them in advance of the extent and purpose of the QA/QI data-gathering.
Q. How can I be sure that my activities are QA/QI and thus exempt from review by an IRB or other research ethics board?
This is known to be a challenging issue. There are no hard and fast rules, and it is not uncommon for QA/QI to generate research questions and evolve into a research study. The chart below offers some guidelines; but it is best to consult with the Chair of your research ethics board, who can help you think through the various factors and decide if an application is warranted.
Q. If I’ve been tracking enrollment statistics and related data for purposes of program evaluation, can I later use those data to plan a research study, e.g., to estimate the size and characteristics of the available research sample?
Yes. As long as it doesn’t infringe on the privacy/confidentiality rights of those who contributed the data, most research ethics boards would consider this an acceptable use of the data. Moreover, most biostatisticians would consider it wise practice.
Q. If I gather outcomes data for the purpose of program evaluation, can I later use those data to answer a research question, e.g., can I contribute them as part of a multi-site research study?
If the data were gathered for program evaluation, the program participants did not give informed consent to have their data used or shared in this way. So it’s best to consult with your research ethics board before proceeding. They may ask if the data in question can be linked in any way to the individuals who contributed those data. If the data can be linked back to the individuals, the ethics board will probably require that those individuals agree in writing to this proposed use of their data. Generally speaking, its best to consider at the start of the project whether the data might be used for other purposes, and plan accordingly.
|